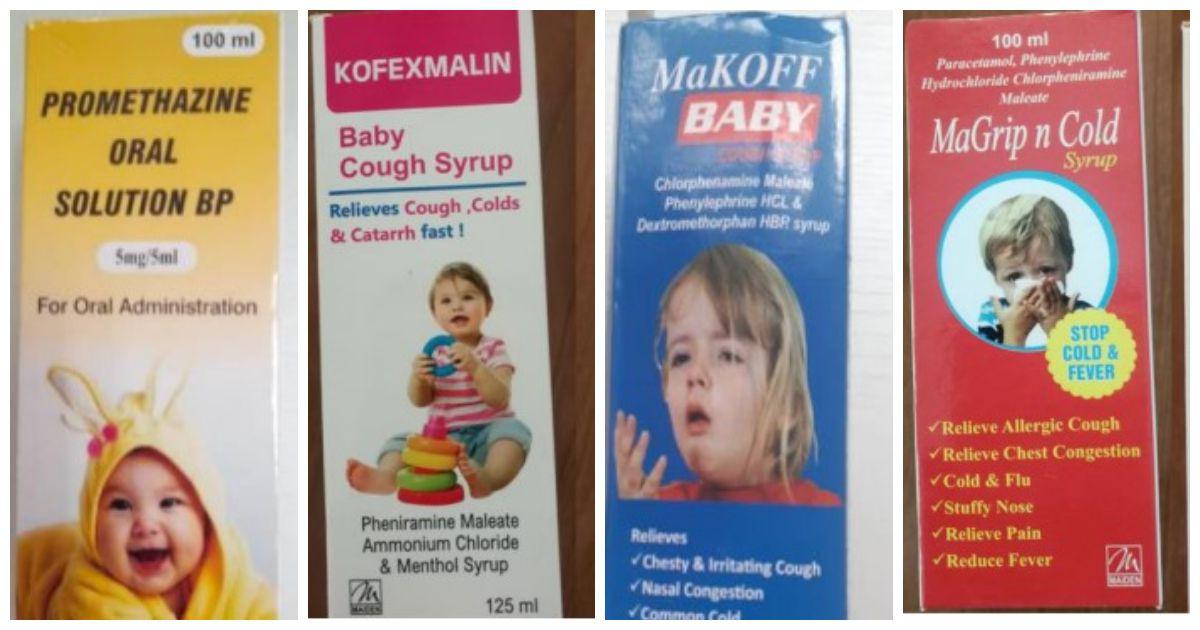
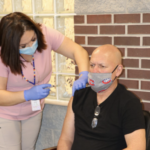
WHO alert on 4 Indian cough syrups containing diethylene glycol, know why is it dangerous?
Cough Syrups by Maiden Pharmaceuticals Banned: Not Long After the World Wellness Organization (THAT) outlawed four India-made cough and cold syrups, the Central Drugs Requirement Control Organisation (CDSCO) launched a probe over the medicines linked to the fatality of 66 kids in The Gambia Made by Haryana-based Maiden Pharmaceuticals Ltd, the four cough as well as chilly syrups that are prohibited by that are– Promethazine Oral Remedy, Kofexmalin Baby Cough Syrup, Makoff Baby Cough Medicine, and Magrip N Cold Syrup. Also Read – WHO Issues Alert, Claims Penetrating Indian Cough Syrup After 66 Children Pass Away in the Gambia.

” The 4 medicines are cough as well as cool syrups produced by Maiden Pharmaceuticals Limited, in India. They failed the examination as they have undesirable quantities of diethylene glycol and also ethylene glycol as pollutants”, the global wellness company had mentioned. According to the tentative results received by the WHO, 4 out of the 23 samples checked have been discovered to contain either Diethylene Glycol/Ethylene Glycol.
WHAT IS DIETHYLENE GLYCOL, AND WHY IS IT DANGEROUS?
- Diethylene Glycol or Ethylene Glycol is considered toxic to the body. When taken in, it can cause kidney and also neurological toxicity. DEG has been associated with several cases of mass poisoning when eaten through medications.
- It is a colorless, virtually odorless, as well as hygroscopic fluid with a sweetish preference. It is miscible in water, alcohol, ether, acetone, and ethylene glycol.
- The poisonous impacts of Diethylene Glycol include abdominal pain, vomiting, diarrhea, failure to pass pee, frustration, a transformed psychological state, as well as severe kidney injury.
- According to a paper in the National Library of Medication 10 DEG mass poisonings have happened over the past 70 years. These mass poisonings were all brought on by DEG-contaminated fluid or lotion drugs. DEG contamination takes place when it is used in medical items rather than a much safer– but more pricey– diluent such as pharmaceutical-grade glycerin.
4 Indian Cough Syrups Banned by WHO

CDSCO EXAMINATION UNDERWAY
Meanwhile, sources close to the matter exposed that the CDSCO has occupied the matter and ordered a detailed investigation against the cough as well as chilly syrups generated and exported to The Gambia by Maiden Pharmaceuticals Limited.
The preliminary query has revealed that Maiden Pharmaceutical Limited is a supplier licensed by the State Medicine Controller for the products under recommendation, as well as holds making approval for these items.
![Zanari CBD Gummies Reviews [NEWEST UPDATE] Every USA Customer Must Know Before Spending!!! Zanari CBD Gummies Reviews [NEWEST UPDATE] Every USA Customer Must Know Before Spending!!!](https://bingnews24x7.com/wp-content/uploads/2025/10/Golden-Fountain-Farms-CBD-Gummies-150x150.png)




![Green Nature Farms CBD Gummies Reviews [Website Fact Check!] Know The Truth Before Buying! Green Nature Farms CBD Gummies Reviews [Website Fact Check!] Know The Truth Before Buying!](https://bingnews24x7.com/wp-content/uploads/2025/09/Golden-Fountain-Farms-CBD-Gummies-150x150.png)



![Zanari CBD Gummies Reviews [NEWEST UPDATE] Every USA Customer Must Know Before Spending!!! Zanari CBD Gummies Reviews [NEWEST UPDATE] Every USA Customer Must Know Before Spending!!!](https://bingnews24x7.com/wp-content/uploads/2025/10/Golden-Fountain-Farms-CBD-Gummies-300x169.png)
![Green Nature Farms CBD Gummies Reviews [Website Fact Check!] Know The Truth Before Buying! Green Nature Farms CBD Gummies Reviews [Website Fact Check!] Know The Truth Before Buying!](https://bingnews24x7.com/wp-content/uploads/2025/09/Golden-Fountain-Farms-CBD-Gummies-300x169.png)



Average Rating